Striatum-Targeted Anhedonia Rapid Treatment (START): a novel transcranial magnetic stimulation paradigm for treating symptoms of anhedonia in depression
2021 Award: $38,280
Anhedonia, the inability to experience pleasure, is notoriously difficult to treat and predicts suicide risk. Thus, novel treatment paradigms are required to address this critical need in psychiatry. In a randomized clinical trial, we investigate a novel treatment paradigm and contribute to scientific knowledge of the neural basis of anhedonia.
Need/Problem: Anhedonia, or the inability to experience pleasure, is a core symptom of major depressive disorder that predicts suicide risk and can be exacerbated by some treatments. Symptoms of anhedonia are notoriously difficult to treat, so novel interventions and a better understanding of the neural basis of anhedonia are required.
Grant Summary: Patients with major depressive disorder and high symptoms of anhedonia will be recruited to participate in a double-blinded placebo-controlled randomized clinical trials that administers non-invasive brain stimulation to the prefrontal cortex in a rapid treatment paradigm.
Goals and Projected Outcomes: The proposed experiment will not only investigate the potential of a novel treatment paradigm to reduce symptoms of anhedonia in major depressive disorder, but also contribute to scientific knowledge of the neural and psychological basis of anhedonia.

Justin Riddle, PhD
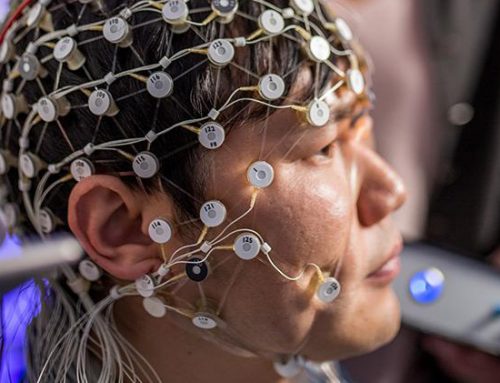
Grant Details: Patients diagnosed with major depressive disorder and elevated symptoms of anhedonia are recruited to participate. During two initial baseline sessions, clinical status is assessed and patients perform a reward-based decision-making task during recording of electrical brain activity (EEG) and high-resolution neuroimaging (fMRI). Two frontostriatal circuits involved in reward-based decision-making that are known to be altered in patients with depression are localized: the dorsolateral prefrontal cortex to dorsal striatum (the “wanting” circuit) and the medial prefrontal cortex to ventral striatum (the “liking” circuit). Then, patients are randomized to receive stimulation to either the dorsolateral prefrontal cortex, the medial prefrontal cortex, or an irrelevant control region (placebo) for five consecutive days of rapid transcranial magnetic stimulation. Finally, clinical status and neural activity during reward-based decision-making are assessed in a two-week follow-up session.